The Shwachman Diamond Syndrome Registry Europe has been newly established in 2020 to set up a registry specifically adapted to the broad spectrum of SDS clinical aspects. It shall help to improve knowledge on SDS with respect to diagnosis, clinical treatments, long term prognosis, assessment of quality of life and risk evaluation of MDS/AML and stem cell transplantation. In addition, it should expand the SDS network and harmonize data management in close cooperation with patient organizations. The initiation of a SDS Registry, separated from the SCNIR (Severe Chronic Neutropenia International Registry), is funded by a grant from the “Sanitätsrat Dr. Emil Alexander Huebner und Gemahlin”- Foundation to take the complexity of SDS into account.
The SDSR-EU monitors the clinical course, treatment and disease outcomes in patients with SDS independent from treatment. Thereby, the registry collects both general and specific clinical information, e.g. on pregnancy, quality of life, malignant transformation, bone marrow transplantation and their outcome after consent of the patients. The data is routinely analyzed and new information is published in peer reviewed journals to inform treating physicians about new findings. Supplementing the database on SDS, biological material like blood or bone marrow samples and smears of SDS patients are collected, tested for genetic abnormalties and stored in the cell bank of the SDSR-EU that may be used for specific relevant research projects on Shwachman-Diamond Syndrome. The SDSR-EU manages patient data in accordance with the applicable data protection regulations, in particular the General Data Protection Regulation (GDPR) of 25th May 2018.
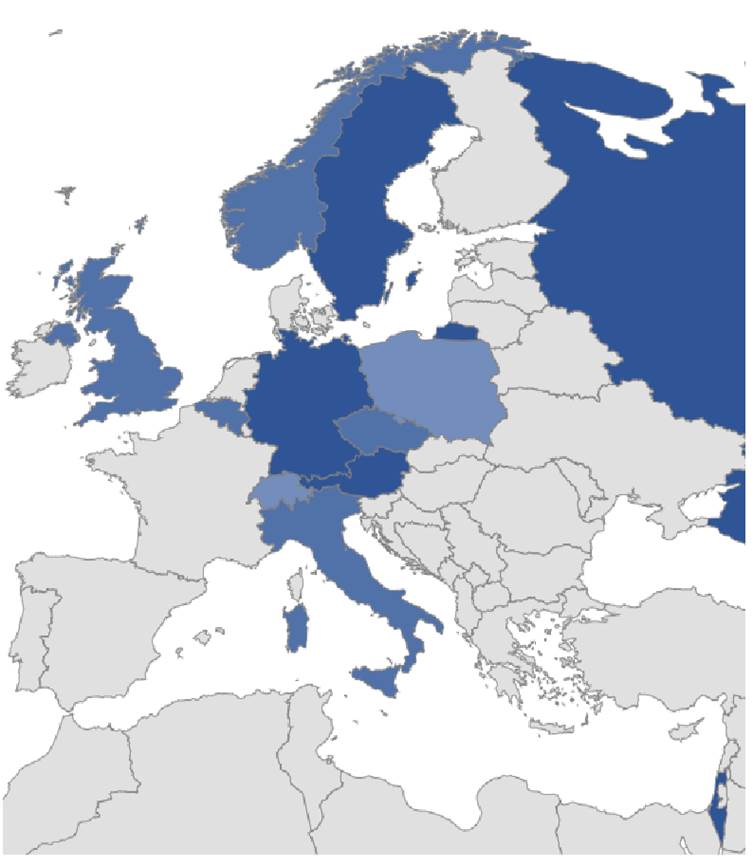
Up to date 110 SDS patients are registered within the SDSR-EU. Participation in the Registry benefits patients, their families, and the physicians, who treat them by providing the most up to date information (to them) on the natural history of SDS and its treatment options.
The SDSR-EU is actively cooperating with pediatricians and hematologists all over Europe. All information arising from the database of the SDSR-EU is disseminated among the members of this network in order to provide an update of the professional skills of the European network partners, which in turn is passed on to other physicians in the participating countries. Annual meetings are held to update all partners on the most current developments and discuss relevant issues on this rare disease.
SDSR-EU Advisory Board Members:
- under construction